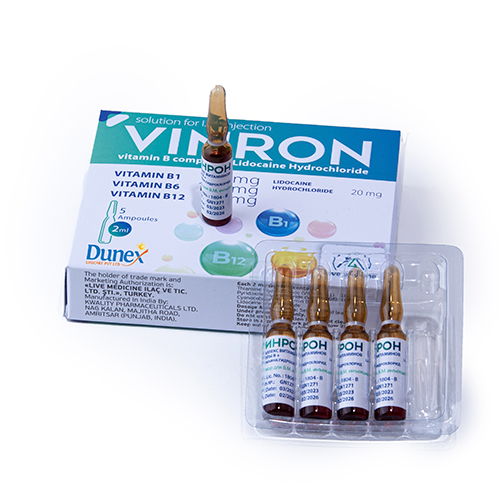
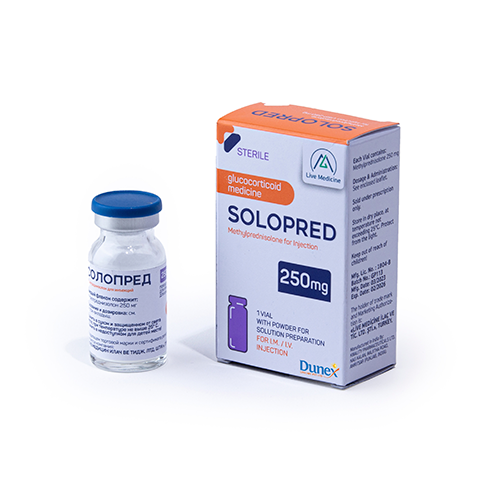
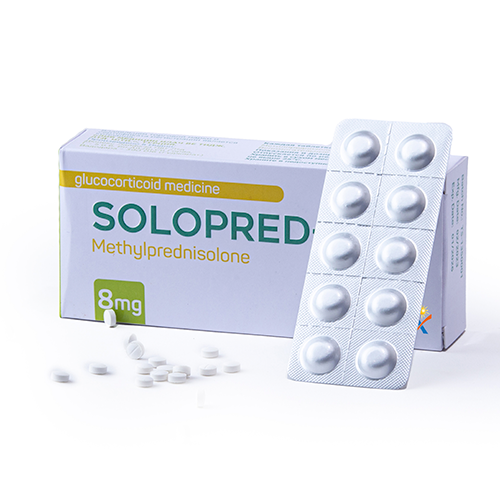
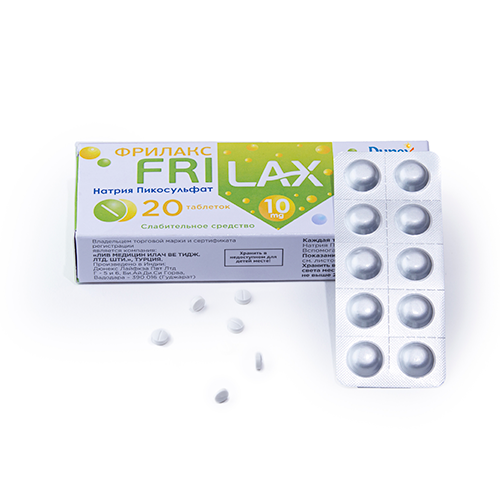
Quality Assurance
Quality assurance in the pharmaceutical products is a continuous process that focuses on the constant review of customer requirements.
microbiology laboratory
It identifies and characterizes microorganisms to support drug discovery, development, and quality control.
Quality control
Quality control verifies and tests the pharmaceutical products at various stages of its life cycle to ensure every product is of the highest quality.